The Safe79 anti-counterfeiting system is the result of ongoing research into new applications for precious metals.

Safe79 is a complete and patented system, which can be applied to multiple materials and it features unique chromatic and thermal characteristics.
This is an innovative system, extremely difficult to replicate and it supplies each single customer with a unique identification code.
The other benefits of the Safe79 anti-counterfeiting system are

The Safe79 system can be applied to all kinds of goods. The coating is homogenous and completely invisible to the naked eye, but it becomes fluorescent when exposed to UV rays. Safe79 can be added to a thread or printed ink without altering the manufacturing process.
Here are some examples of application on various materials:

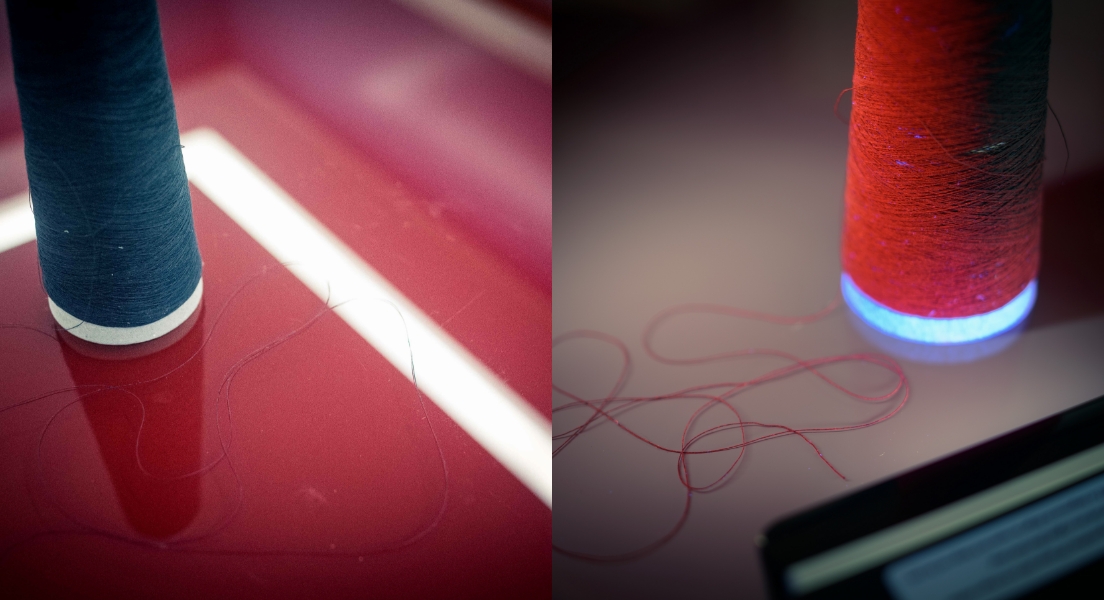



With our system, brand or product counterfeiting can be effectively countered
in various sectors, especially in the luxury industry:




Compila il form per ricevere la presentazione
Fill in the form to receive the presentation